The difference between ester and ether lies mainly in their functional groups: esters contain the –COO group, while ethers contain the –O– group. This single change in structure greatly affects their smell, reactivity, solubility, and boiling points. In simple terms, esters smell fruity, whereas many ethers smell like solvents.
What Are Esters?
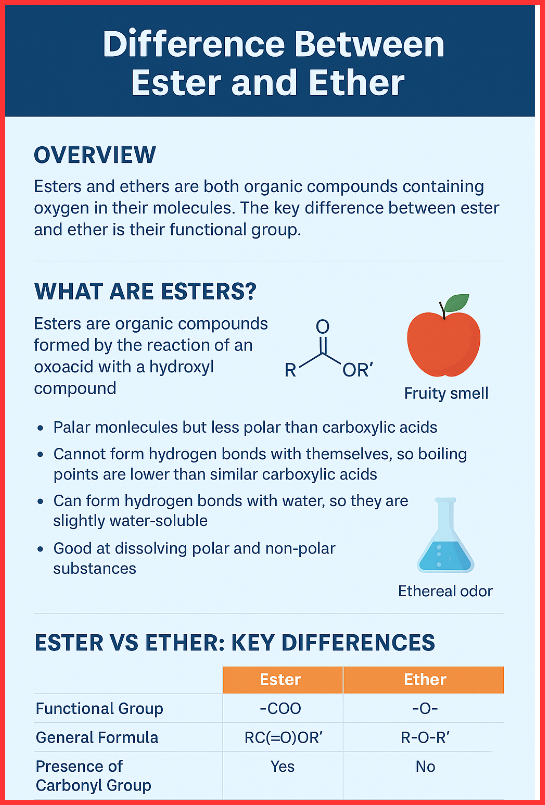
Esters are organic compounds formed when an oxoacid reacts with a hydroxyl compound such as an alcohol or phenol. You can think of an ester as a modified carboxylic acid, where the –OH of the acid is replaced by an alkyl or aryl group.
Structure of an Ester
- Functional group: –COO
- One oxygen is double-bonded to carbon
- One oxygen is single-bonded to carbon
- Carbon is sp² hybridized and forms a trigonal planar geometry
Properties of Esters
- Polar molecules, but less polar than carboxylic acids
- Cannot form hydrogen bonds with themselves, so boiling points are lower than similar carboxylic acids
- Can form hydrogen bonds with water, so they are slightly water-soluble
- Known for their sweet and fruity smell
Examples of Esters
- Ethyl ethanoate (ethyl acetate) – gives fruits like pineapple their pleasant aroma
- Isoamyl acetate – banana smell
- Methyl butanoate – apple fragrance
These pleasant odors make esters widely used in the food, fragrance, and cosmetic industries.
What Are Ethers?
Ethers are organic compounds containing an oxygen atom bonded to two alkyl or aryl groups. They are generally stable, less reactive, and commonly used as solvents.
Structure of an Ether
- Functional group: –O–
- Oxygen is sp³ hybridized
- R–O–R′ bond angle ≈ 104.5° (similar to water)
Properties of Ethers
- Cannot form hydrogen bonds with themselves → lower boiling points than alcohols
- Can form hydrogen bonds with water, so short-chain ethers are water-soluble
- Good at dissolving polar and non-polar substances
- Often have a characteristic “ethereal” odor
Examples of Ethers
- Diethyl ether – common laboratory solvent
- Dimethyl ether – used as a propellant
- Tetrahydrofuran (THF) – industrial solvent
Ester vs Ether: Key Differences (Tabular Form)
Here is a clear, easy-to-understand comparison.
| Feature | Ester | Ether |
| Functional Group | –COO | –O– |
| General Formula | RC(=O)OR′ | R–O–R′ |
| Presence of Carbonyl Group | Yes | No |
| Smell | Fruity, sweet | Strong ethereal/solvent-like |
| Boiling Point | Higher than ethers but lower than alcohols | Lower than esters and alcohols |
| Hydrogen Bonding | Cannot form H-bonds among themselves | Cannot form H-bonds among themselves |
| Solubility in Water | Slightly soluble | Soluble depending on chain length |
| Formation | Reaction of acid + alcohol | Replacement of hydrogen in alcohol by alkyl/aryl |
| Hybridization | Carbon: sp² | Oxygen: sp³ |
| Common Uses | Perfumes, flavors, solvents | Solvents, fuel additives |
Summary: Ester vs Ether
Esters and ethers are both organic compounds containing oxygen, but they differ significantly in structure and behavior.
Esters contain the –COO group, smell fruity, and are widely used in fragrances, cosmetics, and flavoring industries. Ethers contain the –O– group, act as solvents, and have lower boiling points because they cannot form hydrogen bonds with each other.
The essential difference between ester and ether is that esters have a carbonyl-containing functional group (–COO), whereas ethers do not.
This structural difference affects their aroma, solubility, and chemical properties.
Read Next: