The difference between isosmotic hyperosmotic and hypoosmotic lies in their osmotic pressure: isosmotic solutions have equal osmotic pressure, hyperosmotic solutions have higher osmotic pressure, and hypoosmotic solutions have lower osmotic pressure compared to another solution. This difference determines the direction in which water moves across a semipermeable membrane.
What Are Osmotic Conditions?
Osmotic pressure is the force needed to stop water from moving from a low-solute area to a high-solute area through a semipermeable membrane. Biological cells constantly experience osmotic changes, making these terms essential in physiology, chemistry, and medical science.
Key Points:
- Water moves from low solute (low osmotic pressure) to high solute (high osmotic pressure).
- The comparison always involves two solutions.
What is Isosmotic?
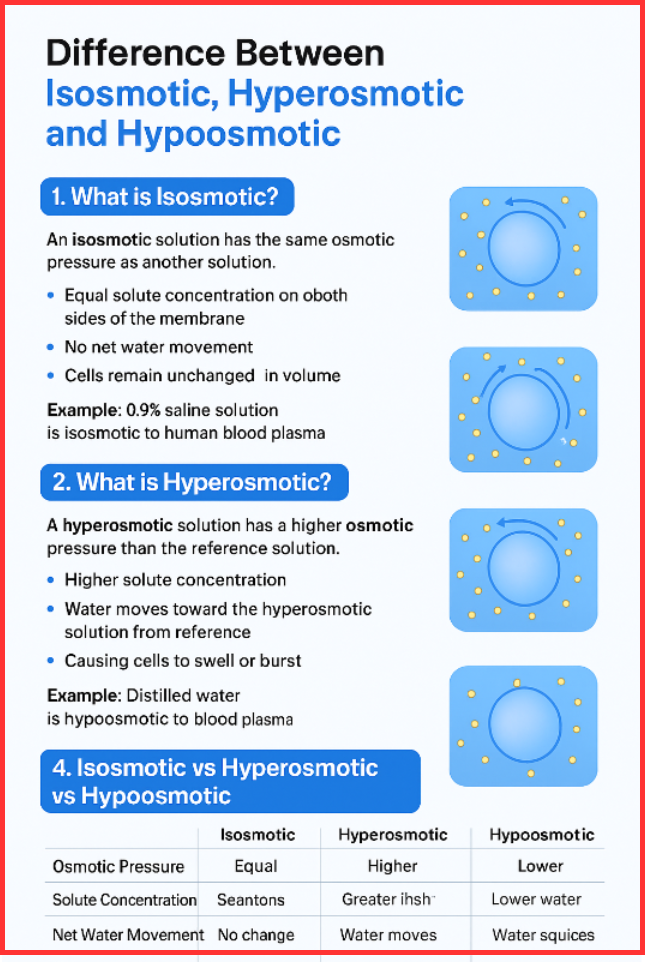
An isosmotic solution has the same osmotic pressure as another solution.
Key Features
- Equal solute concentration on both sides of the membrane
- No net water movement
- Cells remain unchanged in volume
Example
- A 0.9% saline solution is isosmotic to human blood plasma.
What is Hyperosmotic?
A hyperosmotic solution has a higher osmotic pressure than the solution it is compared to.
Key Features
- Higher solute concentration
- Water moves toward the hyperosmotic solution
- Causes cells to shrink due to water loss
Example
- A 2% saline solution is hyperosmotic to human blood.
What is Hypoosmotic?
A hypoosmotic solution has a lower osmotic pressure than the reference solution.
Key Features
- Lower solute concentration
- Water moves into the hypoosmotic solution from the reference
- Causes cells to swell or burst
Example
- Distilled water is hypoosmotic to blood plasma.
Isosmotic vs Hyperosmotic vs Hypoosmotic (Tabular Comaparision)
| Property | Isosmotic | Hyperosmotic | Hypoosmotic |
| Osmotic Pressure | Equal | Higher | Lower |
| Solute Concentration | Same on both sides | Greater in solution | Lower in solution |
| Net Water Movement | None | Water moves out of reference solution | Water moves into reference solution |
| Cell Effect | No change | Shrinks (crenates) | Swells (may burst) |
| Example | 0.9% NaCl vs plasma | 2% NaCl vs plasma | Distilled water vs plasma |
Why These Differences Matter
Understanding these differences is crucial in:
- Medical treatments (IV fluids, dialysis)
- Cell biology (cell volume regulation)
- Food preservation (osmotic pressure inhibits microbes)
- Marine biology (osmoregulation in aquatic animals)
Summary – Difference Between Isosmotic Hyperosmotic and Hypoosmotic
The difference between isosmotic hyperosmotic and hypoosmotic revolves around osmotic pressure:
- Isosmotic → equal osmotic pressure
- Hyperosmotic → higher osmotic pressure
- Hypoosmotic → lower osmotic pressure
These conditions determine the direction of water movement and strongly influence cell stability, making them essential concepts in biology and chemistry. Understanding the difference between isosmotic hyperosmotic and hypoosmotic helps in medical decision-making, laboratory science, and fluid balance studies.
Reference:
1. Helmenstine, Anne Marie. “Osmotic Pressure and Tonicity.” ThoughtCo, Feb. 11, 2020,
2. “Osmotic Pressure.” Chemistry LibreTexts, Libretexts, 5 June 2019,
3. “Osmotic Pressure? What Is Isosmotic? What Is Hyposmotic?” OneClass, 14 May 2019,
Read Next:
- Difference Between Detergent and Chaotropic Agent
- Difference Between Molecular Equation and Ionic Equation
- Difference Between Phosphorylase and Phosphatase
- Difference Between Covalent and Noncovalent Bonds
- Difference Between Aldohexose and Ketohexose
- Difference Between Methyl and Methylene Group (CH3 vs CH2)