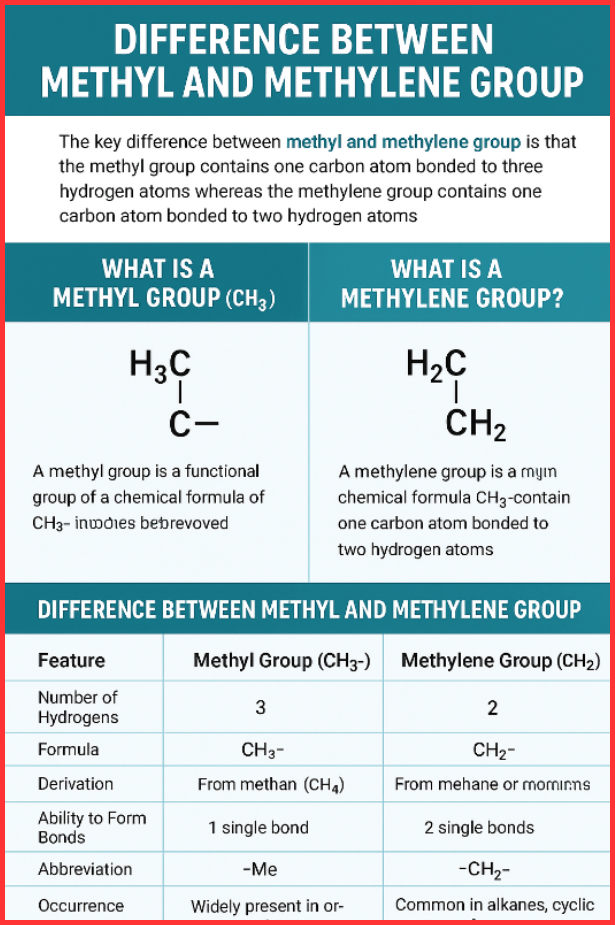
The difference between methyl and methylene group is directly related to the number of hydrogen atoms attached to the carbon atom. A methyl group (CH₃–) has one carbon bonded to three hydrogen atoms, whereas a methylene group (CH₂–) has one carbon bonded to two hydrogen atoms and can form two additional bonds with other atoms. This simple structural difference significantly influences their behavior in organic chemistry.
Understanding the difference between methyl and methylene group is essential in organic chemistry because these two functional groups appear in almost every type of organic compound—from hydrocarbons to complex biomolecules. Despite their similarity, their structure, stability, and reactivity differ, which affects how they participate in chemical reactions.
What Is a Methyl Group? (CH₃–)
A methyl group is a common functional group with the chemical formula CH₃–. It is derived from methane (CH₄) by removing one hydrogen atom. Chemists often abbreviate it as –Me.
Structure
- One carbon atom
- Three hydrogen atoms
- Formula: CH₃–
Forms of Methyl Group
A methyl group can exist in different reactive forms:
- Methyl anion (CH₃⁻) – contains 8 valence electrons
- Methyl cation (CH₃⁺) – contains 6 valence electrons
- Methyl radical (CH₃·) – contains 7 valence electrons
However, these reactive species rarely occur independently; they usually appear as part of larger organic molecules.
Stability and Reactivity
Methyl groups are generally stable and do not react readily with strong acids or bases. Their reactivity largely depends on the neighboring atoms or groups.
Examples of Methyl Group in Organic Compounds
- Methanol (CH₃OH)
- Methyl chloride (CH₃Cl)
- Acetone (CH₃–CO–CH₃)
Oxidation of Methyl Groups
Industrial processes often oxidize methyl groups to produce:
- Alcohols
- Aldehydes
- Carboxylic acids
Example:
Potassium permanganate (a strong oxidizing agent) can convert a methyl group into a carboxylic acid.
What Is a Methylene Group? (CH₂–)
A methylene group has the chemical formula CH₂– and contains:
- One carbon atom
- Two hydrogen atoms
It is usually written as –CH₂– because it forms two covalent bonds with other atoms in an organic chain.
Structure
- One carbon atom
- Two hydrogen atoms
- Formula: CH₂– or CH₂< (indicating two free bonding positions)
Reactivity
A methylene group forms two single bonds in most organic compounds.
When the carbon forms a double bond, the group is called a methylidene group (=CH₂), which is different from a methylene group.
Examples of Methylene Group
- Ethane (CH₃–CH₃) contains methylene units when extended to longer chains
- Cyclohexane, composed of repeating –CH₂– units
- Propane (CH₃–CH₂–CH₃) contains a methylene group in the center
Difference Between Methyl and Methylene Group (Comparison Table)
| Feature | Methyl Group (CH₃–) | Methylene Group (CH₂–) |
| Number of Hydrogens | 3 | 2 |
| Formula | CH₃– | CH₂– |
| Derivation | From methane (CH₄) | From methane by removing two hydrogens |
| Ability to Form Bonds | Forms 1 single bond | Forms 2 single bonds |
| Abbreviation | –Me | –CH₂– |
| Occurrence | Widely present in organic molecules | Common in alkanes, cyclic compounds |
| Reactivity | Highly stable | More reactive than methyl in many cases |
| Example | Methanol, acetone | Propane, cyclohexane |
Summary – Methyl vs Methylene Group
The difference between methyl and methylene group lies in the number of hydrogen atoms and bonding capacity of the carbon atom. A methyl group (CH₃–) contains three hydrogens and forms one bond, while a methylene group (CH₂–) contains two hydrogens and forms two bonds. This structural variation influences their reactivity and role in organic compounds.
Conclusion: Difference Between Methyl and Methylene Group
In conclusion, the difference between methyl and methylene group is defined by structure and bonding: methyl is CH₃– with three hydrogens, and methylene is CH₂– with two hydrogens and two bonding sites. This fundamental distinction governs how each group behaves in organic chemistry and how they appear across organic and biological molecules.
Reference:
1. Helmenstine, Anne Marie. “Methyl Group Definition in Chemistry.” ThoughtCo, Feb. 11, 2020,
2. “Methyl Group.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 21 Nov. 2018,
Read Next: