The Difference Between Lead Acid and Calcium Batteries lies in their electrode composition: lead acid batteries use pure lead electrodes, while calcium batteries use a lead-calcium alloy, resulting in lower self-discharge, longer life, and better cold-weather performance. This direct difference affects their durability, efficiency, and maintenance needs.
Lead acid batteries are traditional rechargeable batteries widely used in cars and backup systems. Calcium batteries are an improved version of lead acid batteries, where calcium is mixed with lead to enhance performance, especially in cold climates.
What Are Lead Acid Batteries?
Lead acid batteries are one of the oldest and most affordable rechargeable battery technologies, introduced by Gaston Planté in 1859.
Key Characteristics of Lead Acid Batteries
- Electrodes: Pure lead plates or lead alloy plates
- High power-to-weight ratio: Good for applications requiring short bursts of high current
- Low energy density: Stores less energy compared to modern batteries
- Heavy structure: Due to lead plates
- Simple charging process: But requires careful monitoring of voltage
- Maintenance required: Especially in flooded lead-acid types
- High water consumption when antimony alloys are used
Why Lead Alloy Is Used
Pure lead is soft. To improve strength, manufacturers add alloying elements such as:
- Antimony: Improves deep cycling but increases water usage
- Calcium: Reduces self-discharge
- Tin & Selenium: Improve conductivity and reduce corrosion
Example Uses of Lead Acid Batteries
- Car starter batteries
- Inverters and UPS systems
- Emergency lighting
- Solar storage (flooded or AGM types)
What Are Lead Calcium Batteries?
Lead calcium batteries are an advanced type of lead acid battery, where calcium is added to the lead grid to enhance lifespan and performance.
Key Characteristics of Lead Calcium Batteries
- Electrodes: Lead-calcium alloy plates
- Low self-discharge rate: Battery retains charge for longer periods
- Longer service life: Due to reduced corrosion
- Performs better in cold temperatures
- Low water loss: Because calcium reduces gassing during charging
- Reduced maintenance: Compared to standard lead acid
Important Note
If lead calcium batteries are overcharged, the plates may grow due to oxidation—reducing their lifespan.
Example Uses of Lead Calcium Batteries
- Modern automotive batteries
- Marine batteries
- Backup power applications
- Solar power bank systems
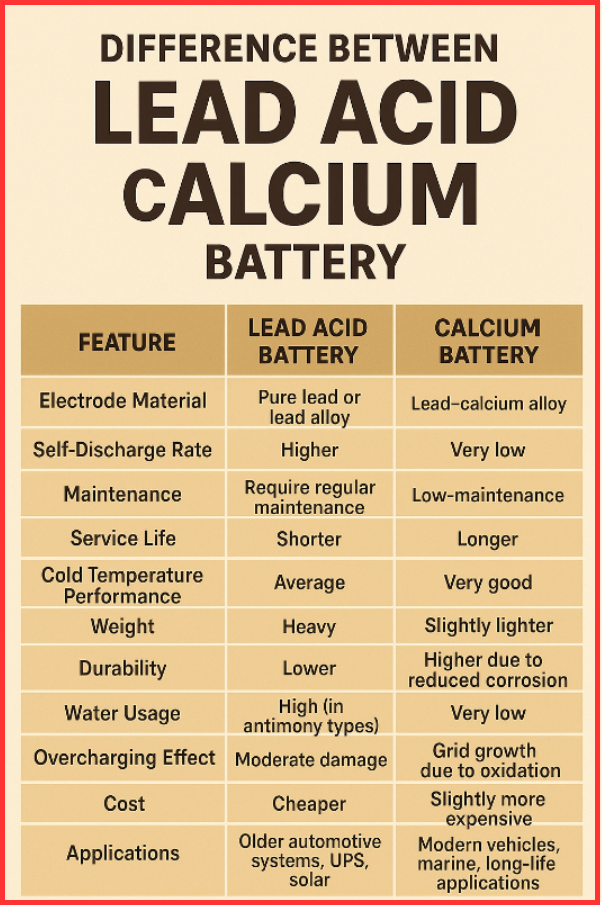
Lead Acid vs Calcium Batteries (Tabular Comparision)
| Feature | Lead Acid Battery | Calcium Battery |
| Electrode Material | Pure lead or lead alloy | Lead–calcium alloy |
| Self-Discharge Rate | Higher | Very low |
| Maintenance | Requires regular maintenance | Low-maintenance |
| Service Life | Shorter | Longer |
| Cold Temperature Performance | Average | Very good |
| Weight | Heavy | Slightly lighter |
| Durability | Lower | Higher due to reduced corrosion |
| Water Usage | High (in antimony types) | Very low |
| Overcharging Effect | Moderate damage | Grid growth due to oxidation |
| Cost | Cheaper | Slightly more expensive |
| Applications | Older automotive systems, UPS, solar | Modern vehicles, marine, long-life applications |
Summary – Lead Acid vs Calcium Batteries
In summary, the Difference Between Lead Acid and Calcium Batteries is mainly based on their electrode composition and performance characteristics. Lead acid batteries use lead electrodes, while calcium batteries use a lead–calcium alloy, giving them better efficiency, lower self-discharge, longer life, and improved cold-weather performance. Therefore, calcium batteries are often preferred in modern automotive and backup power systems.
Reference:
1. “BU-201: How Does the Lead Acid Battery Work?” Charging Lithium-Ion Batteries – Battery University.
2. “Lead–Acid Battery.” Wikipedia, Wikimedia Foundation, 13 June 2018.
Read Next: