- Difference Between Hemolysis and Crenation: Hemolysis occurs when red blood cells swell and rupture in a hypotonic solution, while crenation happens when red blood cells shrink in a hypertonic solution due to osmotic imbalance.
- Hemolysis Explained: In hemolysis, water enters red blood cells by osmosis, causing them to swell, burst, and release hemoglobin; this process is usually irreversible.
- Crenation Explained: Crenation involves water loss from red blood cells, leading to cell shrinkage and a scalloped or spiky appearance, and it is often reversible.
- Osmotic Conditions Comparison: Hemolysis vs crenation highlights opposite osmotic environments—hypotonic solutions cause hemolysis, while hypertonic solutions cause crenation.
- Red Blood Cells Response: Red blood cells show crenation and hemolysis clearly because their membranes are highly sensitive to fluid concentration changes.
- Crenation vs Hemolysis in Practice: Crenation of cell is commonly observed in laboratory experiments, while hemolysis is a major concern in blood transfusions and sample handling.
- Crenation in Dialysis: Crenation in dialysis patients may indicate hypertonic dialysate or electrolyte imbalance and requires careful monitoring.
- Clinical Importance: Understanding hemolysis and crenation helps ensure safe IV fluid use, accurate diagnosis, and effective dialysis and transfusion practices.
The main Difference Between Hemolysis and Crenation is that hemolysis occurs when red blood cells swell and rupture in a hypotonic solution, whereas crenation occurs when red blood cells shrink and develop a scalloped shape in a hypertonic solution. Both processes affect red blood cells due to osmotic imbalance but result in opposite cellular changes.
Introduction to Hemolysis and Crenation
Hemolysis and crenation are important physiological processes related to red blood cells (RBCs) and their response to different osmotic environments. Understanding hemolysis and crenation is essential in biology, medicine, laboratory diagnostics, and clinical practices such as dialysis and blood transfusion.
These processes demonstrate how water movement across the cell membrane impacts cell structure and survival.
What is Hemolysis?
Hemolysis refers to the rupture or destruction of red blood cells when they are placed in a hypotonic solution. In this condition, the solute concentration outside the cell is lower than inside the cell.
Mechanism of Hemolysis
- Water enters the RBC by osmosis
- The cell swells excessively
- The cell membrane bursts
- Hemoglobin is released into the surrounding fluid
This process is usually irreversible, making hemolysis clinically significant.
Causes of Hemolysis
- Hypotonic IV fluids
- Incompatible blood transfusion
- Certain infections and toxins
- Mechanical damage during blood collection
Hemolysis is often discussed in comparison as hemolysis vs crenation to highlight osmotic differences.
What is Crenation?
Crenation is the process in which red blood cells shrink when exposed to a hypertonic solution, where the solute concentration outside the cell is higher than inside.
Mechanism of Crenation
- Water moves out of the RBC
- Cell volume decreases
- The membrane forms spiky or scalloped edges
- The cell appears shriveled
A crenation cell often retains membrane integrity and may recover if returned to an isotonic solution.
Crenation of Cell: Key Points
- Occurs in hypertonic environments
- Often reversible
- Commonly observed in laboratory experiments
Crenation vs Hemolysis: Key Differences
| Feature | Hemolysis | Crenation |
| Osmotic Condition | Hypotonic solution | Hypertonic solution |
| Water Movement | Water enters the cell | Water leaves the cell |
| Cell Size | Cell swells | Cell shrinks |
| Cell Appearance | Cell bursts | Scalloped or spiky edges |
| Reversibility | Irreversible | Often reversible |
| Outcome | Cell destruction | Cell deformation |
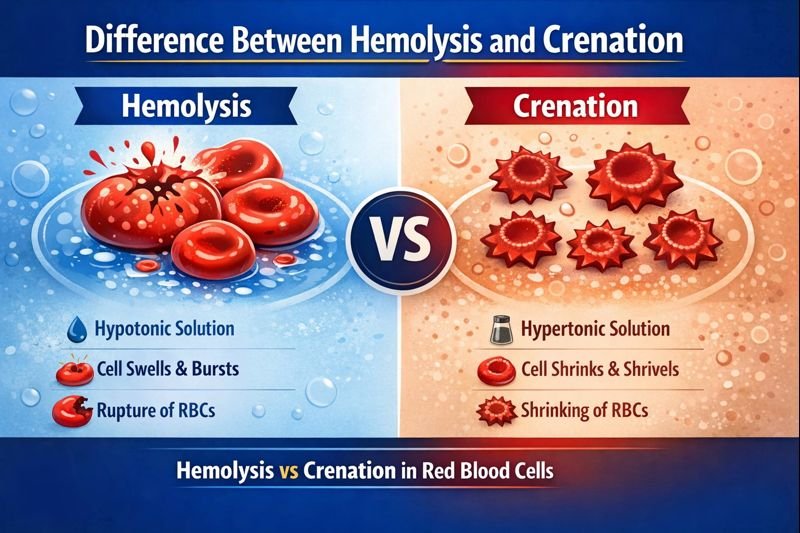
Hemolysis vs Crenation in Red Blood Cells
In red blood cells crenation and hemolysis, osmotic pressure plays a central role. RBCs lack a nucleus and organelles, making them especially sensitive to fluid changes.
- Hemolysis vs crenation demonstrates opposite osmotic effects
- RBC membrane integrity determines survival
- Laboratory slides often show clear visual differences
Crenation Hemolysis or No Change: Solution Types
| Solution Type | Effect on RBC |
| Hypotonic | Hemolysis |
| Hypertonic | Crenation |
| Isotonic | No change |
This concept is crucial when evaluating crenation hemolysis or no change in clinical samples.
Crenation in Dialysis
Crenation in dialysis can occur if the dialysate becomes hypertonic. This causes water loss from RBCs, leading to crenated red blood cells.
Crenation in Dialysis Patient
- May indicate electrolyte imbalance
- Can affect oxygen transport
- Requires careful fluid monitoring
Clinical Importance of Hemolysis and Crenation
Understanding crenation and hemolysis is essential in:
- Blood transfusions
- IV fluid administration
- Dialysis management
- Microscopy and pathology
- Diagnosis of blood disorders
Incorrect osmotic conditions can damage red blood cells and compromise patient safety.
Conclusion
In conclusion, the Difference Between Hemolysis and Crenation lies in the osmotic environment and the resulting behavior of red blood cells. Hemolysis vs crenation highlights how hypotonic solutions cause RBC rupture, while hypertonic solutions lead to cell shrinkage. A clear understanding of hemolysis and crenation, including crenation vs hemolysis, is vital for students, healthcare professionals, and laboratory technicians to ensure accurate diagnosis and safe clinical practice.
Reference:
1. “Hemolysis.” Encyclopædia Britannica, Encyclopædia Britannica, Inc.
2. Mary, McMahon. “What Is Crenation?” Wise Geek. 26 May 2022.
Read Next:
- Difference Between Ingroup and Outgroup in Biology
- Difference Between Cyst and Oocyst
- Difference Between Chemotaxis and Diapedesis
- Difference Between Isosmotic Hyperosmotic and Hypoosmotic
- Difference Between Male and Female Sternum
- Difference Between Dextrose, Dextrin, and Dextran
- Difference Between Junctional and Idioventricular Rhythm